When the subject of a TV show includes the use of deadly toxins, radioactive materials, or biological or chemical agents, you can almost be sure Special Effects artists will make them look a lot more interesting than they are in real life. This is because the vast majority of chemical compounds are colorless, odorless, tasteless and could easily be substituted with a glass of water or a spoonful of salt. Since that doesn't look exciting or menacing, they tend to get totally unrealistic spruce-ups.
Radioactive elements tend to have a Sickly Green Glow, despite the fact that only radium paint does this in Real Life. And even then, it's the zinc sulfide in the paint reacting to high-energy alpha radiation that causes the pale green glow, not the radioactive element itself. Pure radium is an ordinary-looking silvery metal. Since radium paint was once used to make glow-in-the-dark watch faces and instrument gauges before the health risks became known, "radioactive = glowing green" has stuck in the public mind.
For that matter, acid is almost always depicted green and frequently boiling too. While it's true that acid solutions in the real world can vary widely in color from green to red to everything in-between, the acids most often used for modern industrial purposes (sulfuric and hydrochloric) are colorless and clear. Furthermore, boiling them would not be a tremendously useful or wise thing to do. Incidentally, skin contact with most industrial acids will cause a potentially serious and painful chemical burn; but it won't dissolve someone instantly to the bone.
Note, however, that there are several kinds of Deadly Gas in Real Life that are colorful — most notably, fluorine (yellow), chlorine (green), and bromine (red-brown), though bromine is a liquid at room temperature.
Electricity, too, is more colorful and dramatic than in the real world, even more so in animation. Anything electrically charged, such as an electric fence, is more likely than not to light up the surrounding area with crawling arcs of jagged blue fire accompanied by a sizzling noise, even though in the real world such a display requires a charge of tens of thousands of volts and a separation from an electrical ground that is within rather narrow limits. If a person on TV is being electrically shocked, there will almost always be an extremely impressive display of buzzing and crackling arcs of flickering blue fire and perhaps even a display of X-Ray Sparks.
While mains electricity at 100-250 volts is more than capable of killing a human being in the right circumstances, it isn't nearly enough for impressive displays of St. Elmo's Fire.
The center of a nuclear reactor does glow, but it certainly doesn't pulse up and down, and again it's not green. The glow is called Cherenkov Radiation![]() and it's a pleasing steady blue color. Of course, it's only safe to view that blue glow through water, at the bottom of a "swimming pool" reactor, if you see it in air, and not on the other side of thick leaded glass, then to quote Winchell Chung
and it's a pleasing steady blue color. Of course, it's only safe to view that blue glow through water, at the bottom of a "swimming pool" reactor, if you see it in air, and not on the other side of thick leaded glass, then to quote Winchell Chung![]() , "...the good news is you can probably live long enough write your last will and testament. If you write very quickly."
, "...the good news is you can probably live long enough write your last will and testament. If you write very quickly."
Dropping a chunk of dry ice into a beaker of water will cause it to appear to boil and give off a ghostly white fog; this isn't all that useful (sometimes small pellets of dry ice are used this way in order to exclude air from a container briefly), but it is a staple of the Mad Scientist Laboratory. If you add a bit of universal indicator to the water, adding the dry ice will make it turn red and start smoking.
Oddly, the one color you don't often see, but is actually common in laboratories, is hot pink. Phenolphthalein turns bright pink in a basic solution, and is frequently used in acid-base titrations. It will also go red in very strongly acidic solutions, though this is seldom used for anything in a laboratory.
Electronics are not exempt from this either—high-tech machines will often be covered with pretty but seemingly pointless blinking LED lights (which old-school computer types refer to as "das Blinkenlights![]() "), glass pipes full of glowing energy, and meaningless screens displaying bright symbols. Modern technology, especially things that need to be covert, don't usually have these; with the exception of MP3 players.
"), glass pipes full of glowing energy, and meaningless screens displaying bright symbols. Modern technology, especially things that need to be covert, don't usually have these; with the exception of MP3 players.
However, if you go into a lab that works with transition metals, it will be very colorful. It's one of their noted properties.
Gratuitous Laboratory Flasks are the standard container for Technicolor Science in its liquid form; expect to see plenty of examples of the Labcoat of Science and Medicine there as well. See also Technicolor Toxin and Science Cocktail.
Examples:
- In Lamput, the various potions and serums that are created and used by the docs and their fellow lab workers come in vivid colors.
- Oversaturated World: Group Precipitation: "Passing Time, by Fo ME and Masterweaver": In Masterweaver's section, when science is being discussed, "multicolored test tubes" make an appearance.
- All the Troubles of the World: The various computer parts shown during the opening and closing credits include flashing lights and strange dials. The colours are mostly red, green, and beige.
- Ator 2: Orion the Invincible: Akronos has several containers of colorful liquids in his laboratory.
- Back to the Future plays this straight with the lightning and some effects around the DeLorean, along with showing the plutonium as being bright red.note
- Batman has a shootout scene in a chemical plant, which leads to numerous monets where vats and containers are punctured and spray reds and greens all over the place. Jack Napier also falls into a vat of green liquid.
- In Cats & Dogs, Professor Brody's basement laboratory is wall-to-wall with glass beakers of brightly-colored fluids. (This is for a guy trying to produce a vaccine against dog allergies.)
- Lampshaded in Akira Kurosawa's Dreams. One character notes in "Mount Fuji in Red" that the clouds of radiation were colored by scientists to act as identifiers for certain elements; instead, people just associated them with hideous radiation and refer to them as "Death's Calling Card".
- In Logan, Gabriela's footage of the lab where they experimented on the X-23 subjects features test tubes with colorful contents.
- Madame Curie: Marie and Pierre Curie realize that the stain on their bowl is actually the radium they were trying to isolate when they come back to the lab at night and find it glowing in the dark.
- The Trope Maker here would likely be Fritz Lang's Metropolis. The description of the Mad Scientist's lab in the script stated the laboratory is "as much a place for alchemy as science, a magician's lair."
- Of course, the 'color' part isn't so visible, with the movie being black and white.
- Metropolis also featured the Jacob's Ladder electrical demonstrator, that later became famous as the "science-y" thing in the 1931 Frankenstein.
- In The Neanderthal Man, Professor Groves's workroom contains vials full of different-colored liquid, one of which is bubbling and steaming.
- Played straight in The Nutty Professor. Of course, it was a comedy.
- In Re-Animator, the Re-Agent (it revives the dead) is actually lime-green glowstick liquid.
- In The Rock terrorists threaten San Francisco with VX nerve gas, portrayed as ominous green goo inside glass balls.
- In The Rocky Horror Picture Show, Frank N Furter's lab features a tank that he fills with rainbow-colored chemicals to bring Rocky to life.
- In Scream and Scream Again, the Hollywood Acid in Dr. Browning's facility is a sickly yellow colour.
- Averted in Suicide Squad, where the chemical vats that bleached the Joker and Harley Quinn look off-white.
- Averted in the movie adaptation of Stephen King's Thinner, where a gypsy is threatened with a glass of acid that is actually clear.
- In Wild in the Streets, a teenage Max Frost creates a basement drug lab with test tubes and vials of brightly-colored liquid. He tells his mother a dark green substance is LSD.
- Terry Pratchett's Discworld:
- Averted and lampshaded in Feet of Clay. Vimes is very surprised to find out that arsenic is not green since that is how he imagined a deadly poison.
- A dwarf who succumbs to poison in Thud! does salivate green, however.
- Terry Pratchett is very familiar with this trope: when he was press officer for British Nuclear Fuels, he got used to TV journalists bringing their own green smoke and bubbling test tubes so power plants would look right.
- One of R.A. Salvatore's The Legend of Drizzt novels prominently featured a giant green glowing pool of acid sitting in a cave for no reason. Walking above it was perfectly safe, but fall in and you instantly dissolved. Salvatore, it should be mentioned, was working from the manual he'd been given. Such pools of acid, along with oddly retiring lava, were staples of the Forgotten Realms Underdark campaign setting he'd been commissioned to write for.
- The Legend of Rah and the Muggles has purple haze generated by nuclear fallout. Apparently, the moon can shine through it or something. Yeah...
- The potion in The Strange Case of Dr. Jekyll and Mr. Hyde starts out red and then turns purple before settling on green.
- Done infamously in The Twilight Saga: In her world, driftwood fires are blue. Supposedly because of the salt. In Real Life, the most common salt in seawater ... sodium chloride ... produces yellowish-orange flames, or in other words just about the color you'd expect a wood fire's flames to have anyway. There are salts that can produce blue flames, one example being caesium salts, but sodium is much more common in seawater than caesium is.
- In The Documents in the Case, the crucial forensic test is done with the room darkened, the only source of light being a crystal of common salt placed in the flame of a bunsen burner. It's described as giving a deathly greenish glow, though this is probably artistic licence, since the emission spectrum of sodium is orange-yellow.
- The Applied Phlebotinum in The 4400 is the neurotransmitter Promicin, which is the neon green of a chemlight in color — possibly as a Continuity Nod to its discoverer, Jeffrey Combs, who rose to B-Movie stardom wielding nearly identical glowing syringes in the Re-Animator movies.
- Aliens in the Family: When Snizzy tries to bring a dead frog back to life, she uses plutonium. The plutonium has a yellowish-green glow.
- Lampshaded in Arrow, when they open a briefcase containing glowing blue vials.
Felicity Smoak: Why does every secret formula have to be a color? Whatever happened to good old-fashioned clear?
- Batman (1966) usually follows this trope, especially with endless colorful variations of Knockout Gas.
- Battlestar Galactica. Nuclear missiles always having glowing red tips; although this could be some kind of safety measure so ordnance doesn't get mixed up, even the Cylon nukes have this. Averted though with the disassembled nuclear warhead in Baltar's lab which doesn't glow at all.
- The glowing tips are actually little red lights that are presumably there so the audience can see the missiles at all.
- Subverted subtly in The Big Bang Theory when Leonard walks into the kitchen to find Sheldon working with beakers full of colored liquid. Turns out he was trying to improvise growth plates for bacteria using flavored Jell-O. Truth in Television, sort of, as bacterial growth media often are gelatin or similar substances. It's not clear whether an improvisation with Jell-o would work, but it's not an unreasonable thing to try.
- In Breaking Bad the meth starts out as the colorless crystal it is, but later in the series, Walt changes how he cooks and gets Blue Meth. Presumably, this trope is at least part of the reason for the change. (In Real Life, a blue color as pronounced as that seen in the show would indicate the presence of impurities, which is inconsistent with Walt's insistence on extremely high purity product.)
- Doctor Who:
- "The Age of Steel": The Cybermen's electric shocks fit the "crawling blue fire" description, both when the Cybermen use them as an attack and when they malfunction.
- The Dalek apparatus in "Evolution of the Daleks" has both multi-coloured liquids in all sort of containers and a similar "crawling blue fire" effect for the lightning.
- Also avoided, oddly — and inaccurately — enough, in the TV film Einstein and Eddington. Scientists are shown testing the 'new' poison gas chlorine on a number of doves. The gas is colorless. In real life, chlorine actually is green. That's why it's called "chlorine". However, at normal atmospheric temperatures and pressures it's a fairly pale green, so unless you're looking at a considerable thickness of it in front of a very white background the color could easily be missed.
- The third season premiere of Heroes, almost in the same breath as 90% of Your Brain.
- In The IT Crowd, Richmond looks after the machines in what is presumably the server room, except he has no idea what the machines do, nor what the flashing lights mean.
- In an episode of Just Shoot Me!, Maya gets covered in Toxic Waste while in the sewers and is later found to glow green in the dark. The chemicals are specifically described as "phosphates", none of which have this property in real life.
- The element phosphorus, on the other hand, does at least in its white form. It is also highly toxic and can (and does, though its use is controversial) serve as an incredibly lethal firebomb.
- This property is where the name "phosphorus" comes from, after all.
- And it does so because it's just slowly burns in the air.
- The police station in Lucifer has a lab with lots of beakers full of colourful liquids.
- Sci-Fi Channel original movie Megashark vs. Giant Octopus featured this trope in the We're Doing Science! portions. The scientists are trying to find a solution to the problem posed by, well, look at the title. This seems to involve large amounts of pouring liquids from test tubes into larger test tubes. All of these liquids are colored. What are they pouring? Why are they pouring it? Something sciency, I'm sure! Later they come up with the idea of making pheromones, so it's back to the lab! More colored chemicals are poured! Finally, success is achieved. How do they know? Because they pour two chemicals together and they change color to glowing green! Obviously, this means they have achieved shark and squid pheromones. Right?
- In the old Grenada Sherlock Holmes series Jeremy Brett's Holmes usually had a bunch of these scattered around his desk. Usually, they didn't do anything but bubble in the background, but sometimes they were used as a setpiece for solving a crime, or a bit of comic relief.
- The opening credits
 for short-lived series Free Spirit (1989) (featuring a witch named Winnie helping out a mortal family, including a pre-Buffy Alyson Hannigan) show Winnie using her magic to help the younger son with a chemistry experiment, which involves an elaborate setup with lots of flasks and beakers full of brightly colored liquids.
for short-lived series Free Spirit (1989) (featuring a witch named Winnie helping out a mortal family, including a pre-Buffy Alyson Hannigan) show Winnie using her magic to help the younger son with a chemistry experiment, which involves an elaborate setup with lots of flasks and beakers full of brightly colored liquids.
- Spoofed repeatedly on Dinosaurs. In "Power Erupts", a science fair project is a set of multicolored flasks labeled "red", "blue", "purple" and so on. In "Germ Warfare" a doctor prescribes "blue medicine" for baby Sinclair; when it doesn't work, he switches to orange medicine, "the newest and most powerful color known to science".
- The Tamagotchi virtual pets and their adaptations feature a character named Professor Flask who teaches science class at Tamagotchi School. He has a big beaker of green liquid on his head and two more smaller beakers of red and blue liquid for hands.
- Civilization:
- Comes up in Civilization 4, where the map icon for uranium is a bunch of green rocks. Truth in Television the minor uranium ore mineral autunite
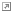 actually is that color. What's more, it glows green under UV light. Also, near what is left of Uravan
actually is that color. What's more, it glows green under UV light. Also, near what is left of Uravan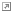 , the abandoned uranium mines can be easily spotted by the clear contrast of the green tailings against the red rock in the area. note
, the abandoned uranium mines can be easily spotted by the clear contrast of the green tailings against the red rock in the area. note
- Civilization 5 avoids Technicolor Science and Green Glowing Rocks by making the icon the radioactivity sign, but now the terrain graphic is green.
- Comes up in Civilization 4, where the map icon for uranium is a bunch of green rocks. Truth in Television the minor uranium ore mineral autunite
- Many futuristic First-Person Shooter games have pits of deadly green acid and radioactive waste, usually glowing green. Doom is the most notable example.
- Earth 2140 is a notable aversion of the radiation subclass. Radioactive fallout doesn't glow green — or anything else. It's invisible and indicated on the minimap with an overlay of black-and-white squares so you can actually tell where it is.
- In the Command & Conquer: Red Alert Series series, radioactive areas are recognizable by the green glow. Though while the first game was serious and grim, the other two are anything but.
- In Command & Conquer: Generals, anthrax comes, in increasing lethality, as green, blue and pink clouds of death. Nuclear radiation, on the other hand, is orange. It still gets reflected by the Chinese Nuke Cannon, which may say "Green... is good." in reference to the Red Alert series' green radiation.
- TimeSplitters Future Perfect plays this for laughs - in one level Cortez and Harry Tipper are trapped in a room filling with green gas, and Tipper comments that green gas is the worst kind.
- In role-playing games with item icons Health potions are often bright red and Mana potions are often bright blue. Poison is green, or if it's particularly deadly, black. Some games may choose other colors. Other types of potions and elixirs come in all manner of colors and shades as well.
- Examples with Red themed health potions and blue themed mana potions include:
- World of Warcraft
- City of Heroes
- Might and Magic
- Diablo
- Dragon Age
- Revenant
- Later Ultima games (the ones where inventory item are shown visually instead of as lists) have red health potions, green poison, blue antidotes, and other multicolored fluids.
- The chemistry set in The Sims yields potions of various bright colors.
- In Half-Life radioactive no-walk zones were Radium-glow green and set off the HEV suit's geiger counter. In Half-Life 2, the same zones are marked by a dark green non-glowing (yet still geiger-crackling) pit of sludge and can be traversed safely with the air-boat.
- In Half-Life 2: Episode One, the Citadel's Reactor Core glows blue because of what we can only assume is Cherenkov radiation. However, it is neither a fusion nor a fission reactor; it was built by Starfish Aliens from Another Dimension and generates dark energy, so any assumptions regarding its mechanics are pointless.
- This trope is played straight and averted in Borderlands. Corrosive elemental weapons and effects are day-glow green, including the expectorants of Spitter Skags. On the other hand, the obviously acidic projectiles from the Soldier, King, and Queen Spiderants are clear fluid in a gel envelope.
- Lost Pig: "Mysterious bubbling liquids in strangely shaped glassware is the heart of alchemy."
- Obsidian contains a puzzle involving test tubes of primary-colored chemicals that need to react in a certain way with two secondary colored chemicals. And each chemical is represented by a geometric shape, with the number of each same-colored test tube corresponding to their "sides". Of course, this example is based in the simulated world of someone's dream, so what need is there for logic?
- In The Feeble Files, Feeble is forced at one point to concoct a potion by mixing differently-colored potions in the right order.
- Risukuma of Puyo Puyo uses explosive beakers in battle, with the liquids in them being in bright colors like blue and green.
- The Strange and Somewhat Sinister Tale of the House at Desert Bridge: Old Man Bill's lab has many flasks and vials of colored liquids. One machine lets you create new items by combining them in different orders.
- According to Tom Siddell's Rant for Gunnerkrigg Court, "...I hereby declare that antigravity is purple and glowy."
- The various Mad Scientist Laboratories in Girl Genius have generally got at least a couple if not many more bottles or/and vials of glowing and brightly colored fluids, often a Sickly Green Glow as in this Castle Heterodyne lab
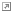 but they show up in other colors as well.
but they show up in other colors as well.
- Narbonic: "I find this line of questioning obscene. What manner of mad scientist neglects his flasks of colored liquid?"
- Parodied in the Homestar Runner toon, "DNA Evidence":
Marzipan: Well, it all started several weeks ago. I came home from my toga-yoga class to find that my house had been broken into and the culprit had left behind some DNA evidence.Strong Sad: What was it? Hair particles? Skin flakes? Blood crispies?Marzipan: No, it was a little test tube just full of green DNA evidence.Strong Sad: Oh! Just like in the movies!
- The eponymous "villain" of Dr. Horrible's Sing-Along Blog has many pieces of colorful liquid-filled equipment in his lab.
- Even the semi-realistic lonelygirl15 fell victim to this, albeit at a point when the realism was really starting to slack off.
- In Italian Spiderman well...
Scientist: It's very red, isn't it?
- Though it wasn't in a vat, The Batman lampshaded this when Batgirl said:
Batgirl: Why is it that deadly chemicals are always green? Why can't they ever be pink?
- The boiling vat of green acid turns up almost too many times to count in Batman: The Animated Series and several times in Teen Titans (2003) (see also No OSHA Compliance).
- Ghost Force: In one episode, Miss Jones is mixing beakers claimed to be water and sulfur trioxide, only one glows green while the other glows bright blue.
- In the second episode of LEGO Friends: The Next Chapter, the friends are trying to perform a science experiment that involves mixing colourful liquids together to produce even more colourful goo. Thanks to their poor teamwork, they end up covered in the goo.
- There are plenty of green glowing things at the nuclear power plant in The Simpsons, including Mr. Burns. In "E-I-E-I(Annoyed Grunt)" Plutonium is shown to be a glowing green fluid, while in real life it's a silver solid (or powder). A carbon rod used to hold a door on a shuttle closed and seen in the opening is shown as a pale lime-green instead of the real-life black, though that could be a coating.
- Rick and Morty in general is brightly colored and tends to get away with this trope, especially concerning Rick's fantastic inventions and other alien technology. This makes seeing a multicolored test tube set become weirdly jarring because it seems so commonplace by comparison. Best example of this is when Doofus Rick is making ovenless brownies for Jerry.
- Brooker's merocyanine
 , or 1-methyl-4-[(oxocyclohexadienylidene)ethylidene]-1,4-dihydropyridine, is an example of a chemical that fits this trope almost exactly; while most colourful chemicals stay the same colour regardless of what they're mixed with, Brooker's merocyanine changes colour depending on the solvent they're dissolved in, a phenomenon called solvatochromism
, or 1-methyl-4-[(oxocyclohexadienylidene)ethylidene]-1,4-dihydropyridine, is an example of a chemical that fits this trope almost exactly; while most colourful chemicals stay the same colour regardless of what they're mixed with, Brooker's merocyanine changes colour depending on the solvent they're dissolved in, a phenomenon called solvatochromism . Dissolved Brooker's merocyanine ranges in colour from bright yellow, to dark blue, to orange, to deep red and even a vivid purple!
. Dissolved Brooker's merocyanine ranges in colour from bright yellow, to dark blue, to orange, to deep red and even a vivid purple!
- Methylene blue
 , commonly used to treat Methemoglobinemia, has a name which is pretty self-explanatory, as it has a remarkably deep royal blue hue that would make a vial of it be right at home in any mad scientist's shelf of lab equipment. Wikipedia's article on it has a fantastic image of a volumetric flask full of the stuff
, commonly used to treat Methemoglobinemia, has a name which is pretty self-explanatory, as it has a remarkably deep royal blue hue that would make a vial of it be right at home in any mad scientist's shelf of lab equipment. Wikipedia's article on it has a fantastic image of a volumetric flask full of the stuff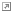 ◊, showing off just how deep its colour is.
◊, showing off just how deep its colour is.
- The University of Leicester once synthesized a krypton compound named "kryptonite" for a lark. It was a colorless crystal with a green light under it, but at least it's fairly harmful (it's a powerful oxidizer), unstable, and contained some radioactive krypton.
- When pure, sulfur is one of the most recognisable non-metals for its distinctive lemon-yellow crystals. When heated, it melts into a red liquid, and when burned, it produces a bright blue flame.
- In some cases, you don't need to look much further for colourful chemistry than your own body. Bruises, for example, will change colour as the body processes the chemicals, from fresh, albeit deoxygenated blood (dark red to purple, depending on how deep the bruise is under the skin), then to bluish green as white blood cells move in to clean up, breaking down haemoglobin into the bile pigment Biliverdin, then to various shades of brown and yellow as the clean up process continues, producing another bile pigment, Bilirubin, then finally back to your natural skin tone as the bruise is completely cleaned up and healed.
- Inorganic and Organometallic Chemistry involves attaching small molecules (usually white powders) to the surface of metal ions (usually in clear solutions). The resulting metal complexes are almost always brightly colored and can be anywhere from orange to green to purple depending on the metal and how strongly the chemicals are stuck to it. Admittedly almost all other chemistry involves white powders, clear crystals or colorless solutions.
- Various metal salts, when put in fire, will turn the flame interesting colors. Sodium compounds (like table salt) give yellow, copper salts - blue, and potassium salts - purple. This is also how they make those nifty little pods that turn campfires weird colours. And also how fireworks come in multiple colors: strontium carbonate is for red, calcium chloride for orange, sodium nitrate for yellow, barium chloride for green, copper chloride for blue, a mix of strontium and copper chloride for purple, and pure barium for white.
- One of the first experiments most secondary school students are allowed to do once they have access to Bunsen burners is to set fire to various Group 1 and 2 elements. Magnesium burns with a brilliant white flame, pure sodium a rich yellow, calcium burns orange, and barium burns bright green!
- One easy example of this trope is the "Electric Pickle
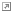 " experiment, where a current passed through a pickle actually gets the pickle to glow, and because the pickle is translucent, the resultant light is bright green. Though ultimately useless beyond demonstrating how electrical charges can move through substances, it is fun to watch. The pickle's been featured in CSI, 1000 Ways to Die and Beakman's World, to name a few instances.
" experiment, where a current passed through a pickle actually gets the pickle to glow, and because the pickle is translucent, the resultant light is bright green. Though ultimately useless beyond demonstrating how electrical charges can move through substances, it is fun to watch. The pickle's been featured in CSI, 1000 Ways to Die and Beakman's World, to name a few instances.
- The cover of the Corning catalogue features flasks, cylinders, and beakers with lovely shades of magenta, violet, and jade. Perhaps someone was running tests on various combinations of food coloring.
- High school Chem books love that display. No surprise. However, it can be recreated (without food coloring, that's cheating) with the right bunch of aqueous solutions. Not that there's terribly much use for it, but it looks cool.
- Magenta/violet/pink could be varying concentrations of potassium permanganate, which is a reasonably common compound used in real laboratories as an oxidizing agent.
- Chalcanthite, a crystal form of Copper(II) sulfate, also known as cupric sulfate, blue vitriol or bluestone has a very distinctive bright blue colour.
- Chromyl chloride, a very toxic compound of Chromium, Chlorine and Oxygen that has a handful of industrial uses, is reddish-brown to blood red in colour, and gives off reddish fumes.
- Some very concentrated acids will give off visible fumes in Real Life, which may be either white or, on occasion, a reddish-brown.
- Red fuming nitric acid is a popular storable hypergolic oxidizer for rocket engines. If you try to store it in a stainless steel container without adding hydrogen fluoride or something as an inhibitor, it will lose performance and turn green via corrosion of the steel.
- Plutonium-238 pellet glow red with heat
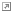 ◊. The glow is thermal radiation, visible due to high temperature of the pellet. The pellet has been sufficiently insulated from its surroundings so that it has heated up to glowy temperature by its radioactive decay.
◊. The glow is thermal radiation, visible due to high temperature of the pellet. The pellet has been sufficiently insulated from its surroundings so that it has heated up to glowy temperature by its radioactive decay.
- More stable plutonium (other isotopes) is silvery-white, though when it is exposed to air it quickly oxidizes, going from dull gray to pinkish-brown to green.
- Radioactive cesium chloride (used in medical radiotherapy, among other things) is a white powder that emits a faint blue glow.
- Some substances actually do change color when another substance is added. Indicators are an example, best known for changing color depending on the acidity of the solution they're in. For instance, bromophenol blue turns a lovely range between yellows and purples if enough acid is added to bring the pH between 3.0 and 4.6. Methyl orange produces some great reds and oranges, and phenolphtalein, a solution of which is colorless at normal pH, becomes a wonderful reddish purple if enough base (for instance Sodium Hydroxide) is added to bring the pH up to about 9.
- This also works with red wine or grape juice. If you add a strong base (such as ammonia water), the red will disappear since the phenolic pigments in red wine and grape juice are pH-sensitive. Just don't try drinking it afterward...
- Triphenylmethyl radical
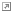 was recognised because solution becomes yellow when it was created.
was recognised because solution becomes yellow when it was created.
- Transition metal compounds are a good example: some familiar examples are potassium dichromate (orange), potassium permanganate (deep purple) and hydrated copper sulfate (blue). Nanoparticle solutions often glow brightly or at least have an interesting color - gold nanoparticle solution is bright pink!
- Aqua regia (a mixture of nitric and hydrochloric acid used to dissolve gold in chemical refining) is clear when made but turns orange in a few seconds due to the formation of nitrosyl chloride and nitrogen dioxide. The resultant chemical produced when gold is reacted with aqua regia, chloroauric acid, is similarly a rich yellow-orange.
- "Electrons are blue" is a common joke explanation for a phenomenon: things with free electrons whizzing about tend to be bright blue.
- This is because of intrinsic properties of free electron gas, which tends to absorb photons with energies in the "reds" range, thus leaving blue light to scatter and reflect. Most organic dyes are molecules with strongly delocalized electron clouds, and tuning the properties of these clouds is used to achieve a great variety of colors.
- An enzyme disorder known as "Porphyria" can cause your pee to turn a bright purple in color.
- Also, very large doses of vitamin C can turn it orange.
- Perhaps many of those gooey green acids seen in Hollywood have been used to dissolve copper? Anyone who's done any analytical chemistry has probably dissolved copper using concentrated nitric acid. If the unknown sample that you're analyzing has contaminants in it (it usually does), dissolving it in the acid produces a very sickly-looking blue-green solution, sometimes cloudy. As a bonus, the stuff emits a torrent of putrid brown nitrogen dioxide as the copper dissolves.
- Also, a common very strong cleaning agent is "chromic acid", which is normally yellowish-brown, but starts to turn bluish-green as the chromium is reduced by use.
- Analytical chemistry is overall a bountiful source of flasks of colored liquids. Many tests for the presence of a compound signal a positive result with an obvious color change, while the Beer-Lambert law
 relates concentration to absorbance of light, making it useful to prepare colored solutions of a substance and stick them in a spectrophotometer. However, as instrumentation has advanced, the field has been moving away from visibly colored solutions to ultraviolet or infrared spectrophotometry, or to solutions so dilute the color is imperceptible.
relates concentration to absorbance of light, making it useful to prepare colored solutions of a substance and stick them in a spectrophotometer. However, as instrumentation has advanced, the field has been moving away from visibly colored solutions to ultraviolet or infrared spectrophotometry, or to solutions so dilute the color is imperceptible.
- A more recent development has been quantum dots
 , metal particles with well-defined sizes; the sizes give rise to specific energy transitions, leading to selective re-emission of photons at specific wavelengths. A very cool way to get specific colors, currently being investigated for use within solar cells.
, metal particles with well-defined sizes; the sizes give rise to specific energy transitions, leading to selective re-emission of photons at specific wavelengths. A very cool way to get specific colors, currently being investigated for use within solar cells.
- More recently, quantum dot-based technologies have found widespread application in flatscreen televisions (branded as "QLED" screens).
- Black bodies
 are objects that reflect little or no light, though they can emit their own light when heated up, and they change color depending on their temperature- going from red, orange, yellow, white, and blue. Incidentally, this is how the temperature of stars are measured.
are objects that reflect little or no light, though they can emit their own light when heated up, and they change color depending on their temperature- going from red, orange, yellow, white, and blue. Incidentally, this is how the temperature of stars are measured.
- Most basic lab work of vegetal physiology involves pigmentary extraction. That means that you end up working with bright green liquids.
- One common demonstration of chlorophyll's photon-absorptive properties involves turning a suspension of chloroplasts from green to deep red.
- "Molecular Biology is mostly mixing lots of clear liquids together in glassware (it's mostly disposable plastic nowadays)." That said, assays for DNA often involve fluorescent compounds (more specifically, chemicals that glow under UV only if they're within the DNA structure, quite cool) and in vivo experiments usually involve tagging things with GFP and it's engineered cousins, all of which fluoresce various colors when exposed to the right wavelength of light. The results are that the labs aren't very colorful, but the image data is.
- Benedict's reagent
 produces a spectrum of colors when exposed to simple sugars like glucose in a solution, going from a clear blue to a green, yellow or red precipitate based on the concentration of the sugars.
produces a spectrum of colors when exposed to simple sugars like glucose in a solution, going from a clear blue to a green, yellow or red precipitate based on the concentration of the sugars.
- Chlorine is quite a colorful element. In its common state, it's a pale yellowish-green gas, but when cooled and under pressure, it becomes a liquid not dissimilar in color to Mountain Dew. The other halogens do similar things; Fluorine is pale yellow, Bromine is a dark reddish-orange, while iodine exists as dark purple crystals that become a deep violet liquid when it melts.
- Neon lights come in multiple colors, though only neon gas itself produces a bright orange glow when introduced to an electric charge. The other colors are made with helium (pink), argon (blue), krypton (white), and xenon (violet). Hydrogen and oxygen both have a lavender glow, though hydrogen is brighter. Mercury vapor is used in ultraviolet black lights, and sodium vapor is used for the bright yellow color of street lights.
- Histology stains can turn cell parts all sorts of different colors, and some stains, like MOVAT's Pentachrome
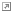 ◊ stain will make you think that you have dropped some acid before heading into the lab. The stains used in the field of histology chemically react with specific cell components and tissue characteristics. This is so that you can turn these structures different colors, making them easier to see for the purpose of microscopic analysis.
◊ stain will make you think that you have dropped some acid before heading into the lab. The stains used in the field of histology chemically react with specific cell components and tissue characteristics. This is so that you can turn these structures different colors, making them easier to see for the purpose of microscopic analysis.
- If different lines on plots in serious science are distinguished by colors, these are often loud colors. More subdued ones are harder to distinguish.
- A popular project recommended in a lot of chemistry books for children involves coating pine cones or other such flammable things with various nitrates in order to make them burn odd primary and secondary colors in a campfire.
- So common is this portrayal of science (especially chemistry) in the media that actual labs will often be decorated with some flasks and beakers containing brightly colored liquids, especially if anyone is filming a documentary or propaganda or any kind of educational video in them. One of Kent Hovind's creationist videos, for instance, features some guy in a white lab coat standing around holding a glass of bright purple liquid with some dry ice fog coming out of it. TV stations around the world like to invoke the trope - if you're in biology or chemistry, even serious news channels demand beakers full of colored liquids in the background when interviewing you.
- As far as electronics go, it's common for gaming PCs and peripherals to have colorful lighting. Custom water-cooling setups can use colored water in the tubes.
- Traditionally, pharmacists would have carboys of coloured liquids
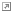 in their shop windows, so that a largely illiterate population could tell what sort of shop it was.
in their shop windows, so that a largely illiterate population could tell what sort of shop it was.
- An aversion: As Cameron says during the 11th annual Desert Bus for Hope, experiments in organic chemistry should never produce a bright red oil.
 Usually, they involve yellow, light brown, or colorless liquids, or white crystals instead.
Usually, they involve yellow, light brown, or colorless liquids, or white crystals instead.

